Abstract
Introduction: Eltrombopag (EPAG), an oral thrombopoietin receptor agonist, has been shown to be effective in raising platelet count (PLTC) and reducing bleeding in patients with chronic immune thrombocytopenia (cITP) in various clinical trials. Clinical data show that plasma exposure of EPAG is higher in East Asians, and a reduced initial dose of 25 mg is effective in Chinese patients. This 3-stage study (NCT01762761) was designed to evaluate the efficacy, safety, and tolerability of EPAG in previously treated adult Chinese patients with cITP (PLTC < 30x109/L for ≥ 12 months). Stage 1 results are published in Yang et al (2017). Here we report the long-term efficacy and safety of open-label EPAG treatment for stage 2 of this study.
Methods: This is a phase III, randomized, placebo-controlled study with an 8-week, double-blinded stage 1; a 24-week, open-labeled stage 2; and a prolonged, open-labeled stage 3. In stage 1, patients were randomized 2:1 to receive either EPAG or placebo. All patients who completed stage 1 were invited to join stage 2 and received open-label EPAG. Patients previously treated with EPAG in stage 1 were maintained on the same dose in stage 2 (EPAG-EPAG; E-E group). Patients who earlier received placebo were started on EPAG 25 mg (placebo-EPAG; P-E group). The dose of EPAG was adjusted according to PLTC per study protocol. In stage 3, EPAG treatment was continued until EPAG became commercially available in China. The endpoints were the proportion of patients with PLTC ≥ 30x109/L and ≥ 2 times the baseline PLTC at least once, incidence and severity of bleeding symptoms (World Health Organization [WHO] bleeding scale), proportion of patients who required a protocol-defined rescue treatment, proportion of patients with PLTC ≥ 50x109/L in ≥ 75% of PLTC assessments, total duration of time patients had a PLTC ≥ 50x109/L, maximum period of time with PLTC continuously ≥ 50x109/L, and proportion of patients that reduced or discontinued baseline concomitant ITP medications. Safety assessments were determined through adverse event (AE) reporting and clinical laboratory evaluations.
Results: Overall, 150 patients (mean age, 43.6±14.98 years; female, 112 [74.7%]) who completed stage 1 entered stage 2: 50 in the P-E group and 100 in the E-E group. A total of 129 patients completed stage 2: 4 in the P-E group and 17 in the E-E group withdrew prematurely, mainly due to non-efficacy.
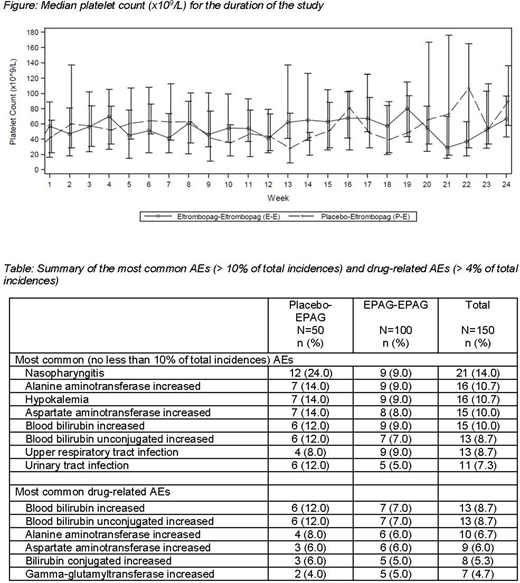
The median PLTC in the E-E group was maintained between 41x109/L and 80x109/L; the P-E group showed similar results with mostly median PLTC > 40x109/L. A majority of patients in both groups (P-E, 90.0%; E-E, 81.8%) achieved PLTC ≥ 30x109/L and ≥ 2 times the baseline PLTC at least once with EPAG treatment (Figure). Moreover, 32% of patients (P-E, 20%; E-E, 38%) achieved PLTC ≥ 50x109/L in ≥ 75% of PLTC assessments. The mean duration that PLTCs were continuously ≥ 50x109/L was 8.34 weeks in total (P-E, 7.59 weeks; E-E, 8.72 weeks).
Both groups showed infrequent bleeding and clinically significant bleeding during stage 2, with continuously decreasing tendency compared to baseline. Clinically significant bleeding generally occurred after week 3 in ≤ 2% of patients in the P-E group and for the entire treatment period in the E-E group.
Protocol-defined rescue treatment was required in 32.0% and 14.0% of patients in the P-E and E-E groups, respectively. Overall, 18.7% required a new ITP medication, 5.3% required platelet transfusion, and 1.3% required increase in concomitant ITP medication from baseline, but none required splenectomy. Among patients who received ≥ 1 ITP medication at baseline, 70.4% in the P-E group and 40.8% in the E-E group reduced or permanently stopped ≥ 1 of their ITP medications; 37.0% and 18.4% permanently stopped all ITP medications, respectively. Overall, 71.3% had ≥ 1 AE; the majority of AEs reported were grade 1 (36%) or grade 2 (13%) in severity, with 11% grade 3 and 4% grade 4 AEs. The most common drug-related AEs included blood bilirubin unconjugated increased (8.7%) and alanine aminotransferase increased (6.7%) (Table). Serious AEs (SAEs) were reported in 11.3% of patients; 3 patients discontinued the stage 2 study medication due to SAEs. None of the AEs were fatal.
Conclusions: Results from the 24-week, open-labeled stage 2 of this study further demonstrated a sustainable long-term efficacy and good tolerability of EPAG with a favorable benefit-risk ratio in Chinese patients with cITP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal